Home page
The Murray lab investigates the genetic regulation of morphogenesis – the cellular mechanisms that help create the myriad forms of the body. Our particular focus is on epithelial plasticity. Although epithelial cells are normally stationary, they can dissociate and become migratory in a process called epithelial mesenchymal transition (EMT). Conversely, migrating cells can cease migration and connect up to form a new epithelium, a process called mesenchymal epithelial transition (MET). Epithelia can also undergo a partial EMT whereby they become motile only at their edges. These mechanisms play crucial roles during development, cancer and wound-healing.
Our lab uses two model systems: the fruit fly, Drosophila melanogaster, and cultured human cells. Drosophila provides an outstanding in vivo model system for biomedical research due to the sophisticated genetic tools available and the high level of conservation between flies and humans. Using genetic screens and genomic profiling techniques in the fly we identify new genes regulating epithelial plasticity and then examine their role in both flies and human cells to identify conserved mechanisms.
Netrins regulation of epithelial plasticity in development and wound healing.
Much of the lab’s work focuses on the role of Netrins, versatile proteins best known as secreted axonal chemoattractants, but also now, strongly implicated in epithelial plasticity events (reviewed in Chaturvedi and Murray, 2021 – Cells, Tissues, Organs, In Press). We first discovered Netrins regulate epithelial cells by promoting the EMT that occurs during wing eversion (Manhire-Heath et al. 2013, Nature Communications). Surprisingly we then found Netrins also play a role in the reverse process, the MET that occurs during formation of the intestinal epithelium in the embryo. Netrins emanating from the visceral mesoderm help guide and polarise the migrating midgut cells so that they can undergo the MET (Pert et al., 2016, Biology Open). We subsequently demonstrated that loss of the Netrin receptor, Neogenin in human CRC cells induces a partial EMT (Chaturvedi et al., 2020, Scientific Reports).
Our current work is focussed on the role of Netrins in wound-healing. In collaboration with Patricia Jusuf’s lab, we’ve found that Netrin expression is rapidly upregulated at the edge of epithelial wounds, in human, zebrafish and fly and are exploring the signaling pathways involved. Thus, in both development and damage, regulation of epithelial integrity involves Netrins.
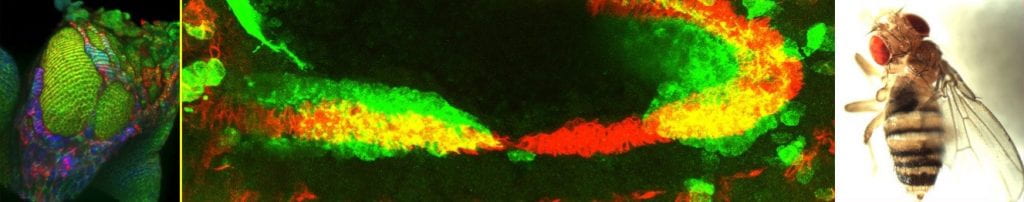
Left to Right: EMT in the peripodial epithelium; Migrating midgut cells (green) in the embryo; Eversion failure due to loss of NetA.
Epithelial homeostasis in the adult midgut.
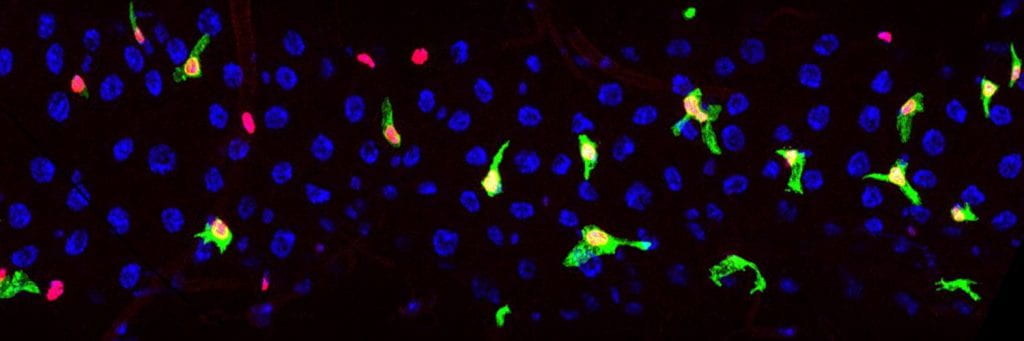
The green cells shown in the image below are enteroblasts. These are motile progenitor cells that, when required, can undergo an MET and differentiate into the absorptive enterocytes that line the gut (large blue cells). We are studying the morphogenetic mechanisms enteroblasts employ to maintain their spatial distribution and incorporate into the epithelium.
GBE-Su(H)-GAL4 driving membrane-targeted GFP and Histone-RFP labels the enteroblasts and newly differentiated enterocytes (image Fionna Zhu).
Dr Mike Murray (Group Leader)
 Mike did his PhD with Paul Whitington at the University of New England. After graduating in 1999 he undertook postdoctoral training with Andrea Brand at the University of Cambridge, UK, and then a second postdoctoral position in Rob Saint’s group at the ANU in Canberra. He then moved to University of Melbourne in 2010 where he established his research program on epithelial-mesenchymal plasticity, and is currently a senior lecturer and MSc(BioSciences) coordinator.
Mike did his PhD with Paul Whitington at the University of New England. After graduating in 1999 he undertook postdoctoral training with Andrea Brand at the University of Cambridge, UK, and then a second postdoctoral position in Rob Saint’s group at the ANU in Canberra. He then moved to University of Melbourne in 2010 where he established his research program on epithelial-mesenchymal plasticity, and is currently a senior lecturer and MSc(BioSciences) coordinator.
Fionna Zhu (PhD candidate)
 Fionna graduated with a Bachelor of Science in 2016 and Master of BioSciences in 2018. Her journey of research started in 2015 when I undertook volunteer work in the lab. In her Masters project, she performed genetic screening to identify novel regulators of MET using the adult Drosophila midgut as a model, specifically the Enteroblast cells (EBs). This project was carried through into her PhD, where she now focuses on studying how the distribution pattern of EBs contribute to damage-induced tissue repair, and more in-depth functional analysis on the role of Septate Junction protein in EB quiescence and MET. Her PhD is supported by the Melbourne Research Scholarship 2019-2022 and Dame Margaret Blackwood Soroptomist Scholarship in 2020.
Fionna graduated with a Bachelor of Science in 2016 and Master of BioSciences in 2018. Her journey of research started in 2015 when I undertook volunteer work in the lab. In her Masters project, she performed genetic screening to identify novel regulators of MET using the adult Drosophila midgut as a model, specifically the Enteroblast cells (EBs). This project was carried through into her PhD, where she now focuses on studying how the distribution pattern of EBs contribute to damage-induced tissue repair, and more in-depth functional analysis on the role of Septate Junction protein in EB quiescence and MET. Her PhD is supported by the Melbourne Research Scholarship 2019-2022 and Dame Margaret Blackwood Soroptomist Scholarship in 2020.
Lachlan Wallace (MSc candidate)
 Lachlan is investigating the role of the Netrin family of proteins in wound healing and epithelial closure. He is co-supervised by Mike Murray and Patricia Jusuf, using both Drosophila and Zebrafish to explore the conserved function of Netrins across animals.
Lachlan is investigating the role of the Netrin family of proteins in wound healing and epithelial closure. He is co-supervised by Mike Murray and Patricia Jusuf, using both Drosophila and Zebrafish to explore the conserved function of Netrins across animals.
Lachlan completed his BSc (Genetics) at the University of Melbourne, and returned to begin his Master’s project in 2020.
Melissa Lai (Honours candidate)
 Melissa is investigating epithelial homeostasis in the adult Drosophila midgut. She is exploring the response of progenitor cells to cell death using an optogenetics approach, using light-activated caspases. Melissa completed her BBmed at the University of Melbourne in 2020.
Melissa is investigating epithelial homeostasis in the adult Drosophila midgut. She is exploring the response of progenitor cells to cell death using an optogenetics approach, using light-activated caspases. Melissa completed her BBmed at the University of Melbourne in 2020.
Georgia Malloy (3rd year Science Research Project undergraduate)
 Georgia is in her third year of a Bachelor of Science with majors in both Genetics and Immunity and infection. She is conducting a small “shelf” RNAi screen for genes that affect fly longevity when challenged with oral Dextran Sodium Sulfate, an established model for inducing gut regeneration. Genes that affect viability will be examined for their effect on enteroblast morphology, distribution and ability to differentiate into enterocytes.
Georgia is in her third year of a Bachelor of Science with majors in both Genetics and Immunity and infection. She is conducting a small “shelf” RNAi screen for genes that affect fly longevity when challenged with oral Dextran Sodium Sulfate, an established model for inducing gut regeneration. Genes that affect viability will be examined for their effect on enteroblast morphology, distribution and ability to differentiate into enterocytes.
Lab Alumni
Dr Vishal Chaturvedi, Postdoc, 2016-2020
Enoch Wong, Msc 2018-2019
Isabelle Lohrey, MSc 2018-2019
Dr Sonia Golenkina, PhD
Jacob Calabria, MSc
Jessica Stott, MSc
Grace Jefferies, MSc
Ben Minarelli, MSc
Caitlyn Perry, MSc
Caitlin Hennessey, MSc
Dr Melissa Pert, PhD
Dr Rose Manhire-Heath, PhD
Chaturvedi, V. and Murray, M. J. (2021) Netrins: evolutionarily conserved regulators of epithelial fusion and closure in development and wound-healing. Cell Tissues Organs. 1–19. http://doi.org/10.1159/000513880
Golenkina, S., Manhire-Heath, R., & Murray, M. J. (2021). Exploiting Drosophila melanogaster Wing Imaginal Disc Eversion to Screen for New EMT Effectors. Methods in Molecular Biology (Clifton, NJ), 2179, 115–134.
Jefferies, G., Somers, J., Lohrey, I., Chaturvedi, V., Calabria, J., Marshall, O. J., et al. (2020). Maintenance of Cell Fate by the Polycomb Group Gene Sex Combs Extra Enables a Partial Epithelial Mesenchymal Transition in Drosophila. G3 • Genes|Genomes|Genetics, 10(12), 4459–4471. http://doi.org/10.1534/g3.120.401785
Chaturvedi V, Fournier-Level A, Cooper HM & Murray MJ. (2019) Loss of Neogenin1 in human colorectal carcinoma cells causes a partial EMT and wound-healing response. Scientific Reports. 1–15.
Golenkina S, Chaturvedi V, Saint R, Murray MJ. (2018) Frazzled can act through distinct molecular pathways in epithelial cells to regulate motility, apical constriction, and localisation of E-Cadherin. PLoS One. 13(3):e0194003.
Murray MJ The Role of Netrins and Their Receptors in Epithelial Mesenchymal Plasticity during Development. (2017) Cells Tissues Organs. 203(2):71-81.
Pert MC, Gan M, Saint R and Murray MJ. (2015) Netrins and Frazzled/DCC promote the migration and mesenchymal to epithelial transition of Drosophila midgut cells. Biology Open. 4(2):1-10.
Murray MJ. (2015) Drosophila models of metastasis. AIMS Genetics 2(1): 25-53. Special Issue: Drosophila models of tumourigenesis.
Doggett K, Turkel N, Willoughby LF, Ellul J, Murray MJ, Richardson HE, Brumby AM. (2015) BTB-Zinc Finger Oncogenes Are Required for Ras and Notch-Driven Tumorigenesis in Drosophila. PLoS One. Jul 24;10(7):e0132987. doi: 10.1371/journal.pone.0132987. eCollection 2015.
Simpson DA, Thompson AJ, Kowarsky M, Zeeshan NF, Barson MS, Hall LT, Yan Y, Kaufmann S, Johnson BC, Ohshima T, Caruso F, Scholten RE, Saint RB, Murray MJ, Hollenberg LC., (2014) In vivo imaging and tracking of individual nanodiamonds in drosophila melanogaster embryos, Biomed. Opt. Express 5, 1250-1261
Manhire-Heath R, Golenkina S, Saint R, Murray MJ, (2013) Netrin-dependent downregulation of the DCC orthologue, Frazzled, is required for the dissociation of the peripodial epithelium in Drosophila. Nature Communications, 4:2790 pp. 1-10, DOI: 10.1038/ncomms3790
Murray MJ, Ng MM, Fraval H, Tan J, Liu W, Smallhorn M, Brill JA, Field SJ, Saint R. (2012a). Regulation of Drosophila mesoderm migration by phosphoinositides and the PH domain of the Rho GTP exchange factor Pebble. Developmental Biology 372, 17-27.
Murray MJ, Southall TD, Liu W, Fraval H, Lorensuhewa N, Brand AH, Saint R. (2012b). Snail-dependent repression of the RhoGEF pebble is required for gastrulation consistency in Drosophila melanogaster. Development Genes & Evolution, 1-8. DOI 10.1007/s00427-012-0414-8
Murray MJ and Saint R, Imaging Pluripotent Cell Migration in Drosophila, in Stem Cell Migration, Methods in Molecular Biology, M.-D. Filippi and H. Geiger, Editors. 2011, Humana Press.
Ebrahimi S, Fraval H, Murray MJ, Saint R, Gregory S. Polo Kinase Interacts with RacGAP50C and Is Required to Localize the Cytokinesis Initiation Complex. Journal of Biological Chemistry (2010) vol. 285 (37) pp. 28667-28673.
Murray MJ and Saint R. Photoactivatable GFP resolves Drosophila mesoderm migration behaviour. Development. 2007. 134: 3975-3983.
Gregory SL, Shandala T, O’Keefe L, Jones L, Murray MJ, Saint R. A Drosophila overexpression screen for modifiers of Rho signalling in cytokinesis. Fly. 2007. Jan; 1(1):e1-e10.
Murray MJ, Davidson CM, Hayward NM, Brand AH. The Fes/Fer non-receptor tyrosine kinase cooperates with Src42A to regulate dorsal closure in Drosophila. Development. 2006. May; (133): 3063-3073.
Smallhorn M, Murray MJ, Saint R. The epithelial-mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development. 2004 Jun;131(11):2641-51.
Murray MJ, Whitington PM. Effects of roundabout on growth cone dynamics, filopodial length, and growth cone morphology at the midline and throughout the neuropile. Journal of Neuroscience 1999 Sep 15;19(18):7901-12.
Murray MJ, Merritt DJ, Brand AH, Whitington PM. In vivo dynamics of axon pathfinding in the Drosophilia CNS: a time-lapse study of an identified motorneuron. Journal of Neurobiology 1998 Dec;37(4):607-21.
Group birding trip to the Western Treatment Plant, Werribee – 27th Nov 2018.
Ausfly poster session, Warburton, VIC
Isabelle Lohrey, busy creating fly/human Hox hybrids.
Awesome edible fly vial, Izzy Lohrey
and an epithelial cake, Izzy Lohrey
Fionna Zhu being awarded Best 2nd Year MSc/PhD Student talk by plenary speaker A/Prof Michelle Arbeitman at the Australian Fly Meeting meeting in Warburton
Escape room solved! Jacob, Jess, Fionna and Vish – Nov, 2017
Group photo at the 2016 Australian Fly Meeting – Alpine Retreat Hotel, Warburton. From back to front. Mike Murray, Jacob Calabria, Jess Stott, Ben Minarelli, Grace Jefferies, Fionna Zhu.
We welcome enquiries from potential students and postdocs.
Email Mike at: murraym@unimelb.edu.au
 Follow us on twitter @murraym_lab
Follow us on twitter @murraym_lab
Dr Michael Murray
 School of BioSciences,
School of BioSciences,
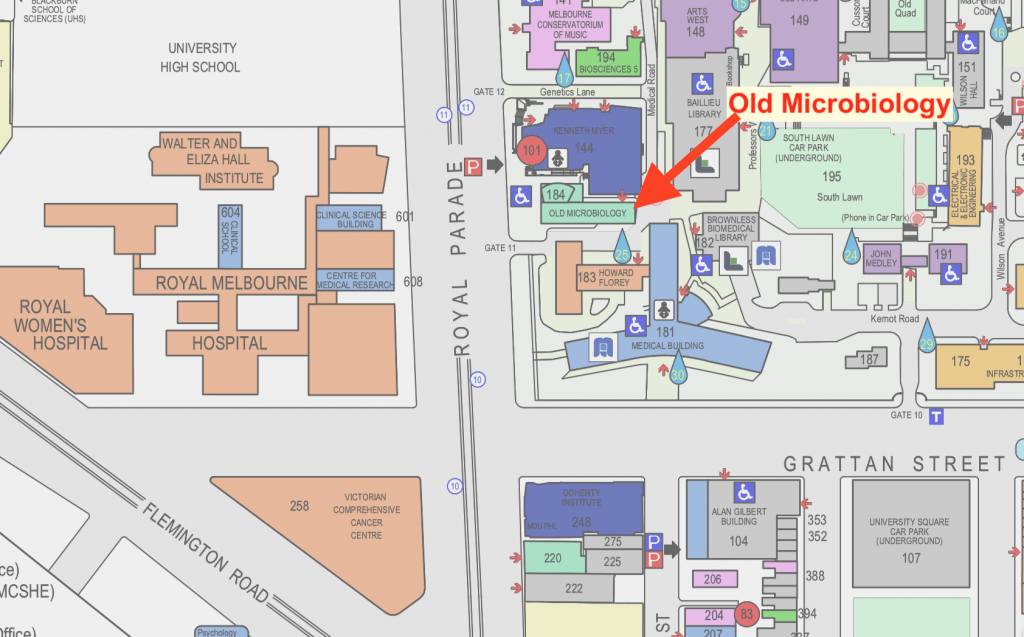
Old Microbiology – Building 184
The University of Melbourne
Royal Parade, 3010 Victoria
Australia